2. Temperature
Temperature is the measurement of a substance’s thermal energy.
So, when we say that a body has a higher temperature than another body,
what we really mean is that its atoms or molecules are moving more quickly.
a) Temperature measurement
We use thermometers to measure temperature. Most thermometers contain liquids which expand and contract when there are changes in temperature.
There are different types of thermometer: mercury, coloured alcohol, or digital.

- Mercury thermometers

Mercury is a liquid metal at room temperature. It changes into a solid at -39 °C,
so mercury thermometers are not useful at temperatures around this value.
An ordinary thermometer consists of a bulb or reservoir, which contains a small amount of mercury, and a long, thin, glass tube. When the temperature increases, the mercury expands. As this expansion only occurs along the length of the tube, it is easy to see with the naked eye.
- Alcohol thermometers
This type of thermometer is very useful
at very low temperatures, because ethanol
changes into a solid at — 114°C.
Alcohol is colourless and it is easy
to dissolve dyes in it, so we use coloured (dyed)
alcohol in alcohol thermometers to make them
easier to read.
Most thermometers that are used to read
outdoor temperatures are made with alcohol.

- Digital thermometers

These thermometers use thermistors. A thermistor is a type of resister thatincreases its electrical conductivity when its temperature increases (the opposite to metals).
Inside the thermometer is a microprocessor that measures the temperature as a function of the resistance of the thermistor. The temperature is displayed on a liquid crystal screen. Nowadays, most thermometers used in the home are digital.
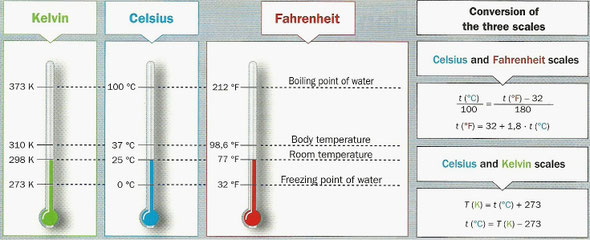
b) Temperature scales
To measure temperature we can use three different scales: the Celsius scale,
the Fahrenheit scale and the Kelvin scale.
- Celsius or centigrade scale (°C)
This is a scale we use to measure normal temperatures.
In this scale the value:
- 0 is the temperature at which water freezes in conditions
of normal atmospheric pressure.
- 100 is the temperature at which water boils in conditions
of normal atmospheric pressure.
The interval between these two values is divided into one hundred equal parts;
each of the parts corresponds to one degree Celsius (1°C).
- Kelvin or absolute scale (K)
This is the real or physical scale that is directly linked to the movement
of particles. The kelvin (K) is the unit of temperature in the International
System of Units (S. I.)
The value 0 in this scale corresponds to the temperature at which
there is no movement in particles: when a substance’s thermal energy is zero.
Absolute zero or zero kelvin (0 K) indicates the natural limit of temperatures:
lower temperatures are not possible, because temperature is the measurement
of the movement of particles.
On this scale, water freezes at around 273 K, so 0°C = 273 K.
The divisions of this scale are the same size as those of the Celsius scale,
so the temperature at which water boils is 100°C = 373 K.
To convert degrees Celsius into kelvin we have to add on 273.
T (K) = t (°C) + 273
- Fahrenheit scale (°F)
This scale is usually used in Anglo-Saxon countries instead of Celsius scale.
The freezing point of water is 32°F and its boiling point 212°F.
The degrees of this scale are different of those of the Celsius scale.
To convert degrees Celsius into degrees Fahrenheit we have to use
this expression:
t (°C) = t (°F) - 32 / 1.8
Animation: Scales of temperature (Tutorvista)

READING ACTIVITIES
After reading the text, copy and answer the following questions into your notebook:
Remember: you must make complete sentences.
2.1. Answer the questions:
a. What properties of matter do mercury and digital thermometers use?
b. Why must the tube of liquid inside a traditional thermometer be very thin?
c. How many degrees Celsius does absolute zero (0 K) correspond to?
d. What type of thermometer will we use in polar regions where temperatures
can reach -40°C? Why?

CALCULATING ACTIVITIES
After reading the text, copy and answer the following questions into your notebook:
Remember: you must extract data, develop operations and give
a solution.
2.2. Change these temperatures into kelvin (K). Are any of them impossible?
Why?
a) -270°C c) 25°C e) -534°C
b) 14,000°C d) -190°C f) 425°C
 BIOGEOSPHERE
Bilingual Biology and Geology
I.E.S. "J.S.Elcano (Sanlúcar Bda.)
BIOGEOSPHERE
Bilingual Biology and Geology
I.E.S. "J.S.Elcano (Sanlúcar Bda.)






